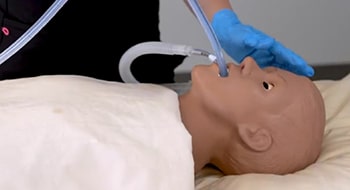
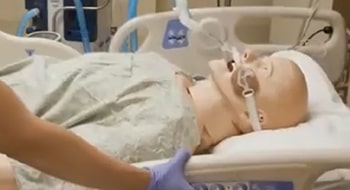
Keeping it Cool
Controlling core temperature
Reducing the likelihood of esophageal injury during RF cardiac ablations
Proactive Esophageal Cooling™
Proactive Esophageal Cooling
The ensoETM® thermal regulating device reduces the likelihood of
esophageal injury during RF cardiac ablation procedures. It is also the
only FDA-cleared device on the market that uses the esophageal
environment for full body temperature control.
Proactive Esophageal Cooling


Mark Metzl, MD, FHRS
Endeavor Health
Learning Center
Reduced Procedure Time and Variability with Active Esophageal Cooling During Radiofrequency Ablation for Atrial Fibrillation
This study utilized advanced informatics techniques to compare procedure duration in patients undergoing radio frequency atrial ablation treated with active esophageal cooling to those treated…
Read MoreEsophageal Heat Transfer for Patient Temperature Control and Targeted Temperature Management
This study presents a novel method to provide efficient patient temperature control for cooling or warming patients.
Read MoreNormothermia Protocols for the Neurologically Impaired Patient
Dr. Joseph Haymore is the Senior Nurse Practitioner on the Neurocritical Care Unit at the University of Maryland and Assistant Professor at the University of Maryland School of Nursing. In this webinar…
Read MoreLatest Company Updates
Haemonetics Corporation Completes Acquisition of Attune Medical
Haemonetics Corporation, a global medical technology company focused on delivering innovative medical solutions to drive better patient outcomes, has completed its acquisition of Attune Medical, the manufacturer of the ensoETM® proactive esophageal cooling device.
Study Finds Significant Reduction in Esophageal Injury Rate When Using ensoETM™ During Cardiac Radiofrequency Ablation Procedures
The study, “Atrioesophageal Fistula Rates Before and After Adoption of Active Esophageal Cooling During Atrial Fibrillation Ablation”, set out to compare the rate of AEFs before and after the adoption of proactive esophageal cooling.
Attune Medical’s ensoETM® Granted FDA De Novo Marketing Authorization to Reduce the Likelihood of Ablation-related Esophageal Injury Resulting from Radiofrequency Cardiac Ablation Procedures
The FDA based its decision on pre-clinical studies ….
Read More This site is intended for US products only.
This site is intended for US products only.